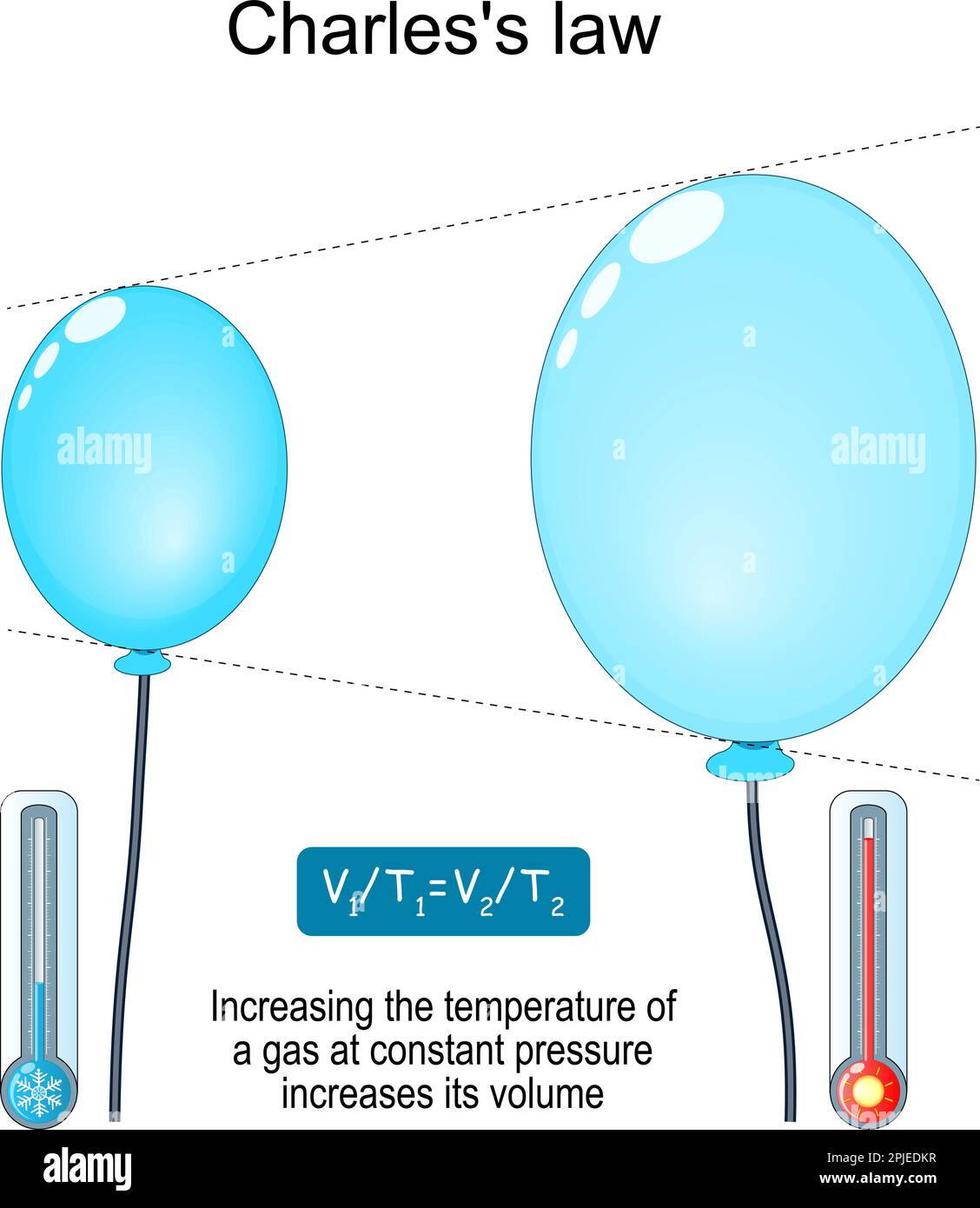
Gas Pressure Increase With Temperature . increasing the temperature of a gas increases the pressure and the energy of the gas particles. as the pressure on a gas increases, the volume of the gas decreases because the gas particles are forced closer together. a temperature increase of \ (1k\) = a temperature increase of \ (1^ {\circ}c \). these examples of the effect of temperature on the volume of a given amount of a confined gas at constant pressure are true. these examples of the effect of temperature on the volume of a given amount of a confined gas at constant. This is a temperature scale where.
from www.alamy.com
a temperature increase of \ (1k\) = a temperature increase of \ (1^ {\circ}c \). This is a temperature scale where. these examples of the effect of temperature on the volume of a given amount of a confined gas at constant. as the pressure on a gas increases, the volume of the gas decreases because the gas particles are forced closer together. increasing the temperature of a gas increases the pressure and the energy of the gas particles. these examples of the effect of temperature on the volume of a given amount of a confined gas at constant pressure are true.
Charles's law. relationship between volume and temperature. Increasing
Gas Pressure Increase With Temperature This is a temperature scale where. as the pressure on a gas increases, the volume of the gas decreases because the gas particles are forced closer together. a temperature increase of \ (1k\) = a temperature increase of \ (1^ {\circ}c \). This is a temperature scale where. these examples of the effect of temperature on the volume of a given amount of a confined gas at constant pressure are true. increasing the temperature of a gas increases the pressure and the energy of the gas particles. these examples of the effect of temperature on the volume of a given amount of a confined gas at constant.
From infinitypipesystems.com.au
Back to Basics What is air pressure? Infinity Pipe Systems Gas Pressure Increase With Temperature these examples of the effect of temperature on the volume of a given amount of a confined gas at constant. as the pressure on a gas increases, the volume of the gas decreases because the gas particles are forced closer together. increasing the temperature of a gas increases the pressure and the energy of the gas particles.. Gas Pressure Increase With Temperature.
From courses.lumenlearning.com
Relating Pressure, Volume, Amount, and Temperature The Ideal Gas Law Gas Pressure Increase With Temperature This is a temperature scale where. as the pressure on a gas increases, the volume of the gas decreases because the gas particles are forced closer together. these examples of the effect of temperature on the volume of a given amount of a confined gas at constant pressure are true. increasing the temperature of a gas increases. Gas Pressure Increase With Temperature.
From www.slideserve.com
PPT Pressure, Volume, Temperature The Gas Laws PowerPoint Gas Pressure Increase With Temperature a temperature increase of \ (1k\) = a temperature increase of \ (1^ {\circ}c \). these examples of the effect of temperature on the volume of a given amount of a confined gas at constant pressure are true. as the pressure on a gas increases, the volume of the gas decreases because the gas particles are forced. Gas Pressure Increase With Temperature.
From slidetodoc.com
The relationship between temperature and volume How Volume Gas Pressure Increase With Temperature increasing the temperature of a gas increases the pressure and the energy of the gas particles. these examples of the effect of temperature on the volume of a given amount of a confined gas at constant. a temperature increase of \ (1k\) = a temperature increase of \ (1^ {\circ}c \). This is a temperature scale where.. Gas Pressure Increase With Temperature.
From www.edplace.com
Understand Gas Pressure Worksheet EdPlace Gas Pressure Increase With Temperature these examples of the effect of temperature on the volume of a given amount of a confined gas at constant pressure are true. This is a temperature scale where. increasing the temperature of a gas increases the pressure and the energy of the gas particles. as the pressure on a gas increases, the volume of the gas. Gas Pressure Increase With Temperature.
From www.sciencephoto.com
Gay Lussac's pressuretemperature gas law, illustration Stock Image Gas Pressure Increase With Temperature these examples of the effect of temperature on the volume of a given amount of a confined gas at constant. as the pressure on a gas increases, the volume of the gas decreases because the gas particles are forced closer together. a temperature increase of \ (1k\) = a temperature increase of \ (1^ {\circ}c \). This. Gas Pressure Increase With Temperature.
From 2012books.lardbucket.org
The Behavior of Real Gases Gas Pressure Increase With Temperature This is a temperature scale where. a temperature increase of \ (1k\) = a temperature increase of \ (1^ {\circ}c \). these examples of the effect of temperature on the volume of a given amount of a confined gas at constant. these examples of the effect of temperature on the volume of a given amount of a. Gas Pressure Increase With Temperature.
From med.libretexts.org
2.3 Gaseous Exchange Mechanism Medicine LibreTexts Gas Pressure Increase With Temperature a temperature increase of \ (1k\) = a temperature increase of \ (1^ {\circ}c \). as the pressure on a gas increases, the volume of the gas decreases because the gas particles are forced closer together. these examples of the effect of temperature on the volume of a given amount of a confined gas at constant pressure. Gas Pressure Increase With Temperature.
From www.slideserve.com
PPT GASES PowerPoint Presentation, free download ID6567281 Gas Pressure Increase With Temperature increasing the temperature of a gas increases the pressure and the energy of the gas particles. a temperature increase of \ (1k\) = a temperature increase of \ (1^ {\circ}c \). as the pressure on a gas increases, the volume of the gas decreases because the gas particles are forced closer together. This is a temperature scale. Gas Pressure Increase With Temperature.
From www.slideserve.com
PPT The Ideal Gas Equation PowerPoint Presentation, free download Gas Pressure Increase With Temperature increasing the temperature of a gas increases the pressure and the energy of the gas particles. these examples of the effect of temperature on the volume of a given amount of a confined gas at constant. as the pressure on a gas increases, the volume of the gas decreases because the gas particles are forced closer together.. Gas Pressure Increase With Temperature.
From www.tec-science.com
Pressure and temperature theory of gases) tecscience Gas Pressure Increase With Temperature these examples of the effect of temperature on the volume of a given amount of a confined gas at constant. increasing the temperature of a gas increases the pressure and the energy of the gas particles. these examples of the effect of temperature on the volume of a given amount of a confined gas at constant pressure. Gas Pressure Increase With Temperature.
From www.alamy.com
Charles's law. relationship between volume and temperature. Increasing Gas Pressure Increase With Temperature a temperature increase of \ (1k\) = a temperature increase of \ (1^ {\circ}c \). as the pressure on a gas increases, the volume of the gas decreases because the gas particles are forced closer together. these examples of the effect of temperature on the volume of a given amount of a confined gas at constant. This. Gas Pressure Increase With Temperature.
From engineerexcel.com
Pressure Temperature Graphs Explained EngineerExcel Gas Pressure Increase With Temperature a temperature increase of \ (1k\) = a temperature increase of \ (1^ {\circ}c \). as the pressure on a gas increases, the volume of the gas decreases because the gas particles are forced closer together. This is a temperature scale where. these examples of the effect of temperature on the volume of a given amount of. Gas Pressure Increase With Temperature.
From saylordotorg.github.io
Effects of Temperature and Pressure on Solubility Gas Pressure Increase With Temperature these examples of the effect of temperature on the volume of a given amount of a confined gas at constant pressure are true. these examples of the effect of temperature on the volume of a given amount of a confined gas at constant. a temperature increase of \ (1k\) = a temperature increase of \ (1^ {\circ}c. Gas Pressure Increase With Temperature.
From www.teachoo.com
Effect of Temperature to Change State of Matter Teachoo Science Gas Pressure Increase With Temperature This is a temperature scale where. a temperature increase of \ (1k\) = a temperature increase of \ (1^ {\circ}c \). these examples of the effect of temperature on the volume of a given amount of a confined gas at constant pressure are true. these examples of the effect of temperature on the volume of a given. Gas Pressure Increase With Temperature.
From www.youtube.com
11.6 The Combined Gas Law Pressure, Volume, & Temperature YouTube Gas Pressure Increase With Temperature these examples of the effect of temperature on the volume of a given amount of a confined gas at constant pressure are true. as the pressure on a gas increases, the volume of the gas decreases because the gas particles are forced closer together. This is a temperature scale where. a temperature increase of \ (1k\) =. Gas Pressure Increase With Temperature.
From engineerexcel.com
Pressure Temperature Graphs Explained EngineerExcel Gas Pressure Increase With Temperature a temperature increase of \ (1k\) = a temperature increase of \ (1^ {\circ}c \). increasing the temperature of a gas increases the pressure and the energy of the gas particles. This is a temperature scale where. these examples of the effect of temperature on the volume of a given amount of a confined gas at constant.. Gas Pressure Increase With Temperature.
From ecampusontario.pressbooks.pub
9.2 Relating Pressure, Volume, Amount, and Temperature The Ideal Gas Gas Pressure Increase With Temperature a temperature increase of \ (1k\) = a temperature increase of \ (1^ {\circ}c \). increasing the temperature of a gas increases the pressure and the energy of the gas particles. these examples of the effect of temperature on the volume of a given amount of a confined gas at constant pressure are true. This is a. Gas Pressure Increase With Temperature.